Chemical Properties of Metals
Chemical Properties of Metals: Overview
This Topic covers sub-topics such as Aqua Regia, Electronic Configuration of Elements, Reactivity Series of Metals, Amphoteric Oxides, Reaction of Sodium with Water, Atomic Number of Elements and, Reactions of Metals with Acids
Important Questions on Chemical Properties of Metals
Silver articles gain weight in polluted atmosphere having . Give reason.
Name the most reactive and less reactive metals.
of zinc is treated separately with an excess of
(a). Dilute hydrochloric acid and
(b). Aqueous sodium hydroxide.
The ratio of the volumes of evolved in these two reactions is:
Write three chemical properties of metal by giving one example.
Sodium metal is stored in kerosene. Why?
Is lead less reactive than hydrogen?
Carbon cannot reduce the oxides of or .
Aluminium, even though a reactive metal, does not react with water. Explain why this is so?
Aluminium is a metal of moderate reactivity; It behaves differently from other metals in certain ways. While common metals form basic Metal oxides, aluminium forms amphoteric oxide. Ores of moderately active metals are found in various forms Like chlorides, carbonate, sulphide, etc.
A) Write the chemical formula for amphoteric oxide of aluminium
B) State the name and chemical formula for Ore of aluminium
C) What is the method used to convert sulphide ores of metal to moderate reactivity into their oxides
D) What is the position assigned to moderately active metals In Reactivity series
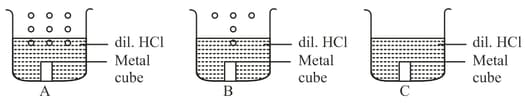
Metal cubes of same size were each dropped in a beaker containing dil. . What are the possible identities of the metal cubes in the beaker?
Does calcium react with nitric acid?
In the reactions,
is more reactive than then how can react with ? Give a brief explanation.
Why sodium is more reactive than lithium?
What is the atomic number of copper?
A metal 'M' when added to a blue-coloured copper sulphate solution, turns it colourless. Is metal 'M' more reactive than copper or less reactive?
How will you show with the help of an experiment that copper is more reactive than silver.
Write the balanced equation of the reaction of zinc with dilute hydrochloric acid and sodium hydroxide solution.
A solution of substance X is used for whitewashing. What is X?
When copper is heated, a layer of is formed on its surface. When we scrape this layer out, would the inner surface be red pure copper?
Both aluminium and iron combine slowly with oxygen at room temperature. Why is this reaction a problem for iron but not for aluminium?
